|
Editorial
The role of cognitive evoked potentials in the diagnosis of neurodegenerative disorders
1 Department of Neurology, University Hospital Center Zagreb, Center for Cognitive Neurology and Neurophysiology, Medical School, University of Zagreb, 10000 Zagreb, Croatia
Address correspondence to:
Natasa Klepac
Department of Neurology, University Hospital Center Zagreb, Kispaticeva 12, 10000 Zagreb,
Croatia
Message to Corresponding Author
Article ID: 100015N06NK2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Klepac N, Skoric MK. The role of cognitive evoked potentials in the diagnosis of neurodegenerative disorders. Edorium J Neurol 2021;7:100015N06NK2021.ABSTRACT
No Abstract
Keywords: Alzheimer’s disease, Binswanger’s disease, Central nervous system, Cognitive evoked potentials
Editorial
Evoked potentials are a widely used neurophysiological method, useful in detection of functional state of different parts of central nervous system (CNS). The usage of the evoked potentials (EP) method is distributed through different areas of medicine, especially in the field of clinical neurophysiology, intra-operative surgical and neurosurgical monitoring, and also in the field of the neuroscience, with a special emphasis on the field of cognitive neuroscience.
One of the great advantages of the EP method is its complete independence of the cultural and education influences [1], and this is especially important in examination of the functionality of the cognitive processes, where objective assessment of the participant’s abilities is important. The evoked response could be obtained also in the situations in which participants do not pay attention to the presented stimulus [2], so the response could be obtained without participant active cooperation.
The method is based on the presentation of the specific stimulus to the participant and the evoked potentials represent the response of the brain and the peripheral CNS to that stimulus [3]. In order to achieve evoked response, specific number of stimuli is presented to the participant and averaging method is used to extract EP response from the recorded signal. To obtain repeatable evoked response, the characteristics of the successive stimuli should be identical.
The EP method is completely non-invasive and it has excellent temporal resolution (~1 ms), and it could present very detailed the temporal sequence of the cognitive and sensory processing. Evoked potential could be endogenous (elicited as a response to specific internal stimulus, e.g., decision making or a reaction to the expectance of the stimulus) or endogenous (elicited as a response to physical characteristics of the external stimulus) [4].
There are different modalities of the evoked potentials, but they are usually divided in two groups: the first group of EP modalities examines the functional integrity of the sensory and motor pathways (visual EP, auditory EP, somatosensory EP, motor EP), and the second group of EP modalities is consisted of methods related to intellectual processing of the presented stimulus (cognitive evoked potentials).
Cognitive evoked potentials provide information about the functionality of the higher cognitive functions, the attention, the memory, and the information processing.
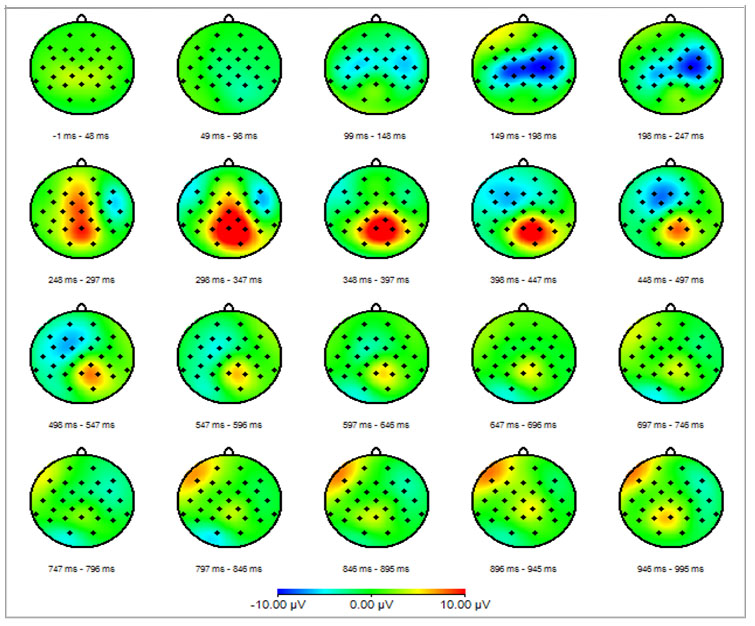
The method presents temporal and spatial distribution of electrical activity of the brain and the peripheral CNS (motor and sensory pathways) elicited as a response to specific stimulus. Most commonly used methods for the presentation of the EP results are waveform presentation (amplitude vs. time) and spatiotemporal mapping (Figure 1).
Results are usually express in the form of components. According to Picton, the component is electrical activity that occurs in the specific part of the brain in the exactly defined period after the stimulus presentation and presents the way on which the brain processes the specific information [3]. The most significant component of the cognitive evoked potentials is the “P300”. P presents positive value of the amplitude and 300 presents the time when the components appears, 300 ms after the presentation of the stimulus.
Cognitive evoked potentials are also called in the literature the “P300.” The “P300” component is introduced in 1965, when Sutton and colleagues presented results of their research [5]. Their results have shown that “P300” appeared only in situations where participants could not predict the upcoming stimulus, while in situations with known stimulus was no “P300” component. At that time, their work has caused a great interest in scientific community and in the years following, the number of research and scientific papers related to “P300,” e.g., cognitive evoked potentials, was getting bigger and bigger. The “P300” component is one of the most studied components in the research of information processing and the selective attention.
Usually used paradigm for cognitive evoked potentials is the “oddball” paradigm. The “oddball” paradigm is based on the presentation of two different stimuli, one that often occurs (“non-target” stimulus) and one that appears rarely (“target” stimulus). During the experiment, participants have a task related to the “target” stimulus. The task could be related to motor reaction—pressing the button after the “target” stimulus; or it could be related to testing the capability of the working memory—counting the number of “target” stimuli. In this way, the ability to discriminate the stimulus, the functional state of the working memory and the decision-making ability are examined and linked to the certain forms of dementia.
During the “oddball” paradigm, the stimulus is presented to the participant. The first step is the sensory processing of the stimulus in order to define the physical characteristic of the stimulus (visual, auditory, somatosensory). After the initial sensory processing, the stimulus is compared with the content of the working memory. If the presented stimulus is the same as the content of the working memory (“non-target” stimulus), then only sensory evoked response is elicited. As opposite, if the presented stimulus is different than the content of the working memory, the “P300” is elicited [6]. The paradigm could also consist of three stimuli, where the third stimulus, “distracting” stimulus, which appears rarely and has similar physical characteristics as the “target” stimulus, and its goal is to improve the difficulty of the task.
The cognitive evoked potentials could be also elicited with the Sternberg’s memory paradigm [7]. The Sternberg’s memory paradigm is based on the presentation of the set of n elements and the “target” stimulus, and the participants has to decide if the “target” stimulus belongs to the previously presented set. The obtained results are reaction time and cognitive evoked potentials. Results have shown that with the increase of the number of elements in the set, the reaction time increases, and also the increase of the number of elements in the set and the affiliation of the “target” stimulus influences the characteristics of the cognitive evoked potentials [8].
Results of the cognitive evoked potentials method are presented through three elements: the latency, the amplitude and the localization of the “P300” component. Also, additional information in the paradigm with motor reaction is the reaction time.
The latency of the “P300” component is related to the speed of classification and processing of the stimulus and it depends on the weight of the task related to the stimulus. It presents the time necessary for the cognitive processing of the stimulus and it is very sensitive temporal measure of the neural activity related to the working memory and selective attention [2],[9],[10]. Difficult classification of the stimulus is related with longer time necessary for stimulus processing which will be expressed as a prolonged latency of the “P300” component. The latency of the “P300” component is negatively correlated with cognitive abilities in healthy participants, and shorter latency is related to higher cognitive abilities [11]. With healthy ageing, the “P300” latency prolongs, as well as in participants with reduced cognitive abilities and with different types of cognitive deficits, like people suffering from different forms of dementia.
The amplitude of the “P300” component presents the intensity of the mental activity necessary for cognitive processing of the presented stimulus. It is proportional to the amount of the attention related to the specific stimulus. Rarely appearing stimuli elicit larger amplitude of the “P300,” as well as stimuli related to decision processing [11]. Difficult processing of the stimulus is related to attenuation in amplitude of the “P300” component due to the theory of “ambiguity” [11]. In ambiguous situations, in which participant is not sure in the characteristics of the presented stimulus, there is reduced amount of the information available for processing and because of that, the intensity of the mental activity required for the processing of the stimulus is reduced which is visible from the attenuation of the amplitude of the “P300” component. Reduced amplitude is also related to the healthy ageing, but also could be an indicator of different forms of cognitive deficits. The variations in the “P300” amplitude are indicators of the degree of the quality of the information processing [12].
The localization of the “P300” component is mostly parietal and one of the indicators for the cognitive deficit could also be the change of the localization. This indicates that neural structures responsible for cognitive processing of the stimulus are not able to complete their function, and some other structures have taken over their function.
The cognitive evoked potentials method is widely used for clinical purposes. Except for the dementia related illnesses, the use of cognitive evoked potentials is important in various psychiatric disorders, such as alcoholism, depression and schizophrenia [9]. Different pathologies related to central nervous system could modify results of the cognitive evoked potentials in a specific way, and because of that, cognitive evoked potentials can provide very useful diagnostic and prognostic information.
Different studies have shown that the latency of the “P300” component is prolonged in patients with dementia in comparison with the age related group of healthy participants [13]. Worsening of the cognitive functions elicits further prolongation of the “P300” latency [14], and because of that the “P300” latency could be potential biomarker of the cognitive abilities of the participants. The correlation between the “P300” latency and the cognitive abilities is also noticed in other neurological conditions not related to dementia and some posttraumatic states [6]. The P300 amplitude could be reduced in participants with dementia in comparison with the age-related group of healthy participants, but there is no correlation with the level of cognitive abilities. Comparison of group of patients with Alzheimer’s disease and healthy controls have shown that the differences between two groups were the most prominent in the basic tasks, with no additional requirements, which shows the value of cognitive evoked potentials in everyday clinical practice [13].
Cognitive evoked potentials and battery of different neuropsychological tests were performed on the same group of patients and results have shown that cognitive evoked potential parameters correlate well with the deficit in domain of language, memory and executive functions indicating that cognitive evoked potential components could be possible biomarkers for neuropsychological deficits [15].
According to some studies, cognitive evoked potentials could be useful in differentiation between cortical (Huntington’s disease, Parkinson’s disease) and subcortical forms of dementia (Alzheimer’s disease, vascular dementia) [6]. Also, the method could be useful in discrimination between the early stage of the Alzheimer’s disease and age related group of healthy participants [13]. Patients in the early stage of the Alzheimer’s disease have reduced amplitude and prolonged latency of “P300” component in comparison with healthy controls.
Research conducted with three groups of participants (AD—group with probable diagnose of the Alzheimer’s disease, MCI—group with mild cognitive impairment and HC—age-related group of healthy controls) showed that at the baseline the “P300” latency was prolonged for AD groups in comparison with MCI and HC group [1]. Also, MCI group had prolonged latency in comparison with HC group. Measurements performed after one year of follow-up showed that only AD and MCI groups had prolonged latency in comparison with results at the baseline, while in HC group the latency did not change significantly. Also, all three groups fulfilled cognitive tests (Mini-Mental State Examination MMSE i Cognitive Abilities Screening Instrument CASI), whose results showed worsening at one year follow-up for MCI and AD group, in correlation with prolongation of the “P300” latency. These results indicate that cognitive evoked potential method could anticipate signs of cognitive deficit, even in situation where clinical indicators are not yet present.
Patients with Parkinson’s disease have reduced “P300” amplitude, and this could also be used for objective assessment of the progression of Parkinson’s disease [16]. Huntington’s disease patients have reduced amplitude or absent response but only in paradigms with visual stimuli. Monitoring of patients with Parkinson’s disease with no signs of cognitive deficit at the baseline has shown that after six years of follow-up the prolongation of the “P300” latency is correlated with the motor progression, which means that cognitive deficit is related with motor disability. This could be explained with the presumption that degeneration of cognitive neural structures is related to degeneration of motor neural structures [17]. Also, group of patients monitored for two years have shown that patients with motor worsening have also prolonged latencies of cognitive evoked potentials, while patients with no motor worsening did not have any statistically significant change in the cognitive components, indicating association between cognitive and motor worsening [18].
Cognitive evoked potentials could also have diagnostic role in patients with stroke [16]. Changes related to “P300” latency could correlate with the level of disabilities caused by stroke. Despite the diagnostic role, the cognitive evoked potentials could also have prognostic role in patients with stroke, and there is a correlation between the parameters of cognitive evoked potentials and functional recovery after few months.
Patients with vascular dementia have difficulties related to discrimination of stimuli and they have prolonged “P300” latency. Also, vascular dementia is related to cortical lesions and it can be expected that patients with this type of dementia have problems with cognitive processing. Studies have shown that patients with vascular dementia had reduced amplitudes and prolonged latencies [19]. Reduced amplitude is related to limited resources available for cognitive processing and this could be caused with vascular lesions and because of that patients with vascular dementia have lower ability of cognitive processing.
Research performed on group of patients with mild cognitive impairment caused with vascular changes, group of patients with subcortical vascular dementia and group of healthy controls have shown that there is statistically significant difference in the latency values between the three groups, pointing that with progression of vascular damage the latency is become more prolonged and that “P300” latency could be indicator of severity of vascular damage [20]. Contrary to that, no statistically significant difference in the “P300” latency was found between the groups of patients with Alzheimer disease, vascular dementia, and mild cognitive impairment, while “P300” amplitude correlated with the degree of cognitive impairment [21].
Comparison of patients with Alzheimer’s disease and patients with subcortical forms of dementia have shown that each group of patients has different deterioration of function of the working memory presented through prolongation of different components of cognitive evoked potentials and that could be explained with different pathogenic mechanisms [22].
Analysis of latency, amplitude, and localization of cognitive evoked potentials performed on group of patients with Parkinson disease, group patients with elderly dementia and group of non-demented patients have successfully classified more than 90% of participants showing the role of cognitive evoked potentials in differential diagnostics [23].
Research performed on three groups of patients (FTD—frontotemporal dementia, AD—Alzheimer’s dementia, and HC—healthy controls) has shown that there was no statistical difference in the “P300” latency between the FTD and HC, but AD group had prolonged latency in comparison with FTD and HC [24]. In the same time, FTD and AD groups have prolonged reaction time in comparison with HC group. These results present usefulness of cognitive evoked potentials in differentiation between different forms of dementia.
Multimodal evoked potentials (cognitive evoked potentials, somatosensory evoked potentials, and visual evoked potentials) were performed on four groups of participants: groups of patients with Alzheimer’s disease (AD), group of patients with Parkinson’s disease (PD), group of patients with Binswanger’s disease (BD), and group of healthy controls [25]. Results have shown that all three groups of patients with dementia have prolonged latency of cognitive evoked potentials, AD and PD groups have prolonged latencies of some components of the somatosensory evoked potentials, and only PD patients have prolonged latencies of visual evoked potentials components. Binswanger’s disease patients have prolonged latencies for all components of the somatosensory evoked potentials. It could be concluded that each group of patients with dementia has specific electrophysiological characteristics and there is a possible role of multimodal evoked potentials in differentiation between different forms of dementia.
SUGGESTED READING
- Babiloni C, Blinowska K, Bonanni L, et al. What electrophysiology tells us about Alzheimer’s disease: A window into the synchronization and connectivity of brain neurons. Neurobiol Aging 2020 85:58–73.
- Tarawneh HY, Mulders WHAM, Sohrabi HR, Martins RN, Jayakody DMP. Auditory electrophysiological assessments of Alzheimer’s disease and preclinical stages: Protocol for a systematic review and metaanalysis. BMJ Open 2020;10(7):e033308.
- Gu L, Zhang Z. Exploring potential electrophysiological biomarkers in mild cognitive impairment: A systematic review and meta-analysis of event-related potential studies. J Alzheimers Dis 2017;58(4):1283–92.
- Kumar A, Foster TC. Neurophysiology of old neurons and synapses. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 10.
REFERENCES
1.
Lai CL, Lin RT, Liou LM, Liu CK. The role of event-related potentials in cognitive decline in Alzheimer’s disease. Clin Neurophysiol 2010;121(2):194–9. [CrossRef]
[Pubmed]

2.
3.
Habek M, Adamec I, Barun B, Crnošija L, Gabeli? T, Krbot Skori? M. Clinical neurophysiology of multiple sclerosis. Adv Exp Med Biol 2017;958:129–39. [CrossRef]
[Pubmed]

4.
Picton TW, Bentin S, Berg P, et al. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology 2000;37(2):127–52.
[Pubmed]

5.
Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science 1965;150(3700):1187–8. [CrossRef]
[Pubmed]

6.
Polich J. Updating P300: An integrative theory of P3a and P3b. Clin Neurophysiol 2007;118(10):2128–48. [CrossRef]
[Pubmed]

7.
Sternberg S. High-speed scanning in human memory. Science 1966;153(3736):652–4. [CrossRef]
[Pubmed]

8.
9.
Polich J. P300 Clinical utility and control of variability. J Clin Neurophysiol 1998;15(1):14–33. [CrossRef]
[Pubmed]

10.
Duncan-Johnson C, Donchin E. The P300 component of the event-related brain potential as an index of information processing. Biol Psychol 1982;14(1–2):1–52. [CrossRef]
[Pubmed]

11.
12.
Vecchio F, Määttä S. The use of auditory event-related potentials in Alzheimer’s disease diagnosis. Int J Alzeimers Dis 2011;2011:653173. [CrossRef]
[Pubmed]

13.
Morrison C, Rabipour S, Knoefel F, Sheppard C, Taler V. Auditory event-related potentials in mild cognitive impairment and Alzheimer’s disease. Curr Alzheimer Res 2018;15(8):702–15. [CrossRef]
[Pubmed]

14.
Katada E, Sato K, Ojika K, Ueda R. Cognitive event-related potentials: Useful clinical information in Alzheimer’s disease. Curr Alzheimer Res 2004;1(1):63–9. [CrossRef]
[Pubmed]

15.
Lee MS, Lee SH, Moon EO, et al. Neuropsychological correlates of the P300 in patients with Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 2013;40:62–9. [CrossRef]
[Pubmed]

16.
Patel SH, Azzam PN. Characterization of N200 and P300: Selected studies of the event-related potential. Int J Med Sci 2005;2(4):147–54. [CrossRef]
[Pubmed]

17.
Hayashi R, Hanyu N, Tamaru F. Cognitive impairment in Parkinson’s disease: A 6 year follow-up study. Parkinsonism Relat Disord 1998;4(2):81–5. [CrossRef]
[Pubmed]

18.
Hayashi R, Hanyu N, Kurashima T, Tokutake T, Yanagisawa N. Relationship between cognitive impairments, event-related potentials, and motor disability scores in patients with Parkinson’s disease: 2-year follow-up study. J Neurol Sci 1996;141(1–2):45–8. [CrossRef]
[Pubmed]

19.
Xu J, Sheng H, Lou W, Zhao S. Approximate entropy analysis of event-related potentials in patients with early vascular dementia. J Clin Neurophysiol 2012;29(3):230–6. [CrossRef]
[Pubmed]

20.
Levada OA, Trailin AV, Kvitka AL, Stolbinskaia OV. P300 potential parameters at the stages of formation of the subcortical vascular dementia in elderly. [Article in Russian]. Lik Sprava 2014;(1–2):60–6.
[Pubmed]

21.
Egerházi A., Glaub T, Balla P, Berecz R, Degrell I. P300 in mild cognitive impairment and in dementia. [Article in Hu]. Psychiatr Hung 2008;23(5):349–57.
[Pubmed]

22.
Muscoso EG, Costanzo E, Daniele O, Maugeri D, Natale E, Caravaglios G. Auditory event-related potentials in subcortical vascular cognitive impairment and in Alzheimer’s disease. J Neural Transm (Vienna) 2006;113(11):1779–86. [CrossRef]
[Pubmed]

23.
Pavarini SCI, Brigola AG, Luchesi BM, et al. On the use of the P300 as a tool for cognitive processing assessment in healthy aging: A review. Dement Neuropsychol 2018;12(1):1–11. [CrossRef]
[Pubmed]

24.
Hedges D, Janis R, Mickelson S, Keith C, Bennett D, Brown BL. P300 amplitude in Alzheimer’s disease: A meta-analysis and meta-regression. Clin EEG Neurosci 2016;47(1):48-55. [CrossRef]
[Pubmed]

25.
Morrison C, Rabipour S, Knoefel F, Sheppard C, Taler V. Auditory event-related potentials in mild cognitive impairment and Alzheimer’s disease. Curr Alzheimer Res 2018;15(8):702–15. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Natasa Klepac - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Magdalena Krbot Skoric - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Natasa Klepac et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.