|
Technical Reports
Minimally invasive endoscopic evacuation of thalamic hemorrhage by the trans-middle temporal gyrus approach
1 MD, Director, Department of Neurosurgery, Hokuto Hospital, Obihiro, Hokkaido, Japan
Address correspondence to:
Akira Tempaku
7-5, Inada-chokisen, Obihiro, Hokkaido 080-0833,
Japan
Message to Corresponding Author
Article ID: 100021N06AT2025
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Tempaku A. Minimally invasive endoscopic evacuation of thalamic hemorrhage by the trans-middle temporal gyrus approach. Edorium J Neurol 2025;10(1):1–6.ABSTRACT
Aims: Thalamic hemorrhage is known to have a poor clinical prognosis. Although surgical removal of hematoma can contribute to early neurological improvement in other type of intracranial hemorrhage, thalamic hemorrhage is sometimes much more difficult to achieve a good outcome by surgery. Less invasive surgical strategies for thalamic hematoma have been well discussed. However, conventional craniotomy via transinsular approach or endoscopic surgery via the ipsilateral trans-frontal or occipital lobe approach is still more invasive due to the long approach distance.
Methods: The endoscopic trans-middle temporal gyrus approach was used in two cases of thalamic hemorrhage. The navigation system was used to mark the puncture point and to guide toward the hematoma. This helped to spare the eloquent area from surgical invasion.
Results: Thalamic hematoma was evacuated by endoscopic surgery through middle temporal gyrus approach. The less invasive and shorter approach method has contributed to achieve the desired result. It has shown the shorter operation time and reduced amount of bleeding in the operation.
Conclusion: The usefulness of trans-middle temporal gyrus approach for endoscopic-assisted hematoma removal of the thalamic hemorrhage is described here. In addition, the anatomical features and practicalities of this approach are discussed.
Keywords: Endoscope, Middle temporal gyrus, Thalamic hemorrhage
INTRODUCTION
Thalamic hemorrhage is associated with impaired consciousness, sensory and motor deficits, aphasia, and cognitive dysfunction [1],[2]. The main cause of thalamic hemorrhage is hypertension, but vascular anomalies such as arteriovenous malformations and moyamoya disease or tumorigenesis sometimes cause it [3],[4]. Although the volume of hematoma is usually small, thalamic hemorrhage is often responsible for the severe deficits [5],[6]. This is because the thalamus contains 5 major functional components, referred to as reticular and intralaminar nuclei, sensory nuclei, effector nuclei, associative nuclei, and limbic nuclei. Impairment of these nuclei results in severe neurological disorders. In addition, the brain stem is located in caudal, the pyramidal tract is lateral, and the ventricles are medial, close to the thalamus. The effects of hematoma expansion and surrounding edema from thalamic hemorrhage easily lead to impaired consciousness and motor paralysis. Prolonged bed rest due to severe neurological disorders is likely to lead to disuse syndrome or aspiration pneumonia. There is also a high risk of complications such as malnutrition or various infections associated with continued medical treatment including intravenous drips. Due to the poor prognosis of neurological function, there is a high likelihood of deterioration in daily functioning, including poor improvement in activities of daily living and increased need for nursing care [5],[7]. Therefore, early evacuation of the hematoma after the onset of thalamic hemorrhage may be desirable to improve the neurological prognosis and activities of daily living [8]. Craniotomy-based evacuation of intracerebral hematoma has not been shown to improve functional outcome [9],[10]. In contrast, minimally invasive hematoma evacuation has been suggested to have a good outcome [11]. However, the thalamus is located deep in the brain, and surgical treatment tends to be highly invasive. Therefore, surgical intervention for thalamic hemorrhage is difficult to achieve a good clinical outcome. To date, the efficacy of this surgery is still controversial [12]. A transcortical approach to the craniotomy from the frontal lobe is likely to be associated with greater brain dysfunction due to the long penetration of the normal frontal lobe to reach the hematoma. Endoscopic surgery via the ipsilateral trans-frontal lobe approach for thalamic hematoma is also usually associated with large brain damage around the trajectory [13]. With transventricular evacuation, it is sometimes difficult to remove the hematoma if it extends to the lateral or caudal part. The trans-sylvian approach involves the pyramidal tracts outside the thalamus and is associated with motor deficits. To avoid these complications, less invasive endoscopic neurosurgery has been widely used to evacuate intracranial hematoma [14],[15],[16],[17],[18],[19],[20]. These reported procedures still have several problems with each other, but they have marked the useful solutions.
Therefore, the endoscopic trans-middle temporal gyrus approach was developed, instead of the conventional surgical craniotomy or endoscopic approach to manage these complicated cases. My cases showed the safety and feasibility of treating thalamic hemorrhage. This trans-middle temporal gyrus approach could preserve neurological function in a minimally invasive manner. In addition, early rehabilitation through rapid improvement of impaired consciousness would contribute to improved prognosis. I would like to discuss the anatomical and functional preserving features of this approach.
PATIENTS AND METHODS
Two patients with thalamic hemorrhage underwent endoscopic surgical hematoma evacuation at the Hokuto Hospital between July 2024 and August 2024. They were treated using the endoscopic trans-middle temporal gyrus approach. The indication for this surgery was that the patient was independent or lightly assisted in activities of daily living (ADL) before the onset of the disease. Patients had severely impaired level of consciousness at presentation, with Glasgow Coma Scale (GCS) ≤ 8.
Surgical treatment was performed under general anesthesia. The head was placed in full lateral rotation position with semi-spine lateral decubitus. One burr hole was perforated on the squamosal suture above the earlobe with a curved liner two-layer skin incision. The superficial temporal artery was preserved in front of the skin incision site. After dura incision and cortical puncture, a 6.8 mm diameter thinner transparent sheath (NeuroPort mini: Hakko, Nagano, Japan) was inserted toward the hematoma with a 2.7 mm diameter rigid endoscope (KARL STORZ Endoscopy Japan, Tokyo, Japan) using a neuronavigation system (StealthStation S7: Medtronic, Dublin, Ireland). Thalamic hematoma was evacuated by aspiration and irrigation with an irrigation and suction device (HOYA Technosurgical, Tokyo, Japan) under endoscopic assistance. Microbleeds from the passing artery or perforators in the hematoma cavity and its wall were brought to hemostasis with monopolar coagulation. After hematoma removal, the burr hole was covered with temporal muscle. Muscle and skin were sutured in layers with absorbable sutures. No drainage tube was placed in the hematoma removed cavity and tract.
CASE SERIES
Case 1
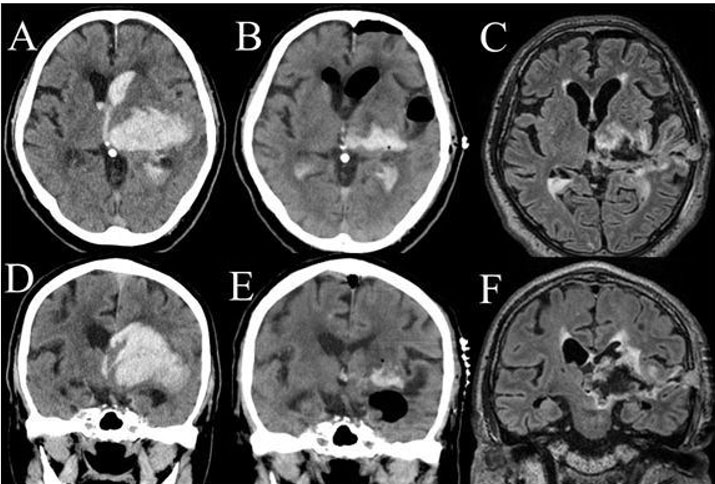
Case number 1 was a 78-year-old man. He had severe level of consciousness (GCS 7) with right hemiparesis. Head computed tomography (CT) showed the lateral type left thalamic hemorrhage (63.7 mL) with ventricular perforation and midbrain invasion of the hematoma (Figure 1A and Figure 1D). Thalamic hemorrhage was classified as posterolateral type by Chung’s classification [21], and as type IIIb hemorrhage by Kanaya’s classification [22]. The intracranial hematoma (ICH) score [23] was 3. The hematoma was removal by endoscopic surgery under general anesthesia (Figure 1B and Figure 1E). The operation time was 60 minutes. The course of endoscopic surgery was shown in Figure 1C and Figure 1E. His level of consciousness was restored to 9 points in GCS. He marked 5 points in modified Rankin Scale (mRS) after one month of operation. His Glasgow Outcome Scale (GOS) was 2.
Case 2
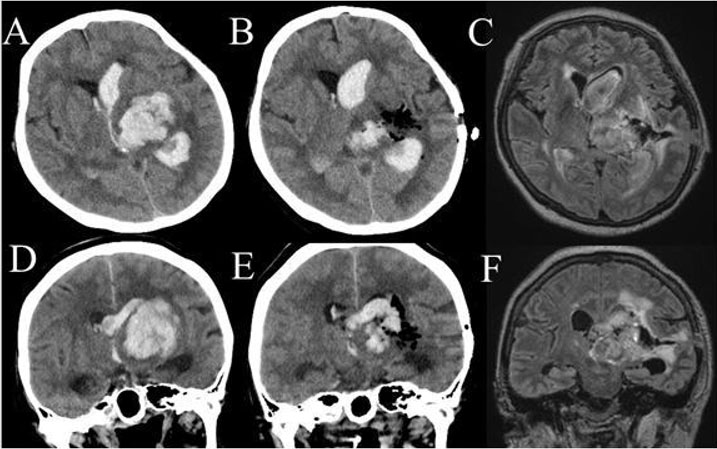
Case number 2 was a 56-year-old woman. She had severe level of consciousness (GCS 7) with right hemiparesis. Head CT showed lateral type left thalamic hemorrhage (38 mL) with ventricular perforation (Figure 2A and Figure 2D). The thalamic hemorrhage was classified as posterolateral type in Chung’s classification, and as type IIb hemorrhage in Kanaya’s classification. The ICH score was 3. Hematoma removal was performed by endoscopic surgery under general anesthesia (Figure 2B and Figure 2E). The operation time was 92 minutes. The course of endoscopic surgery was shown in Figure 2C and Figure 2F. Her level of consciousness was restored to 10 points in GCS. She marked 4 points in mRS after one month of operation. Her GOS was 3.
RESULTS
One male and one female were included. The mean age of the patients was 67 years (ranging from 56 to 78 years). Mean level of consciousness on admission was 7 points with GCS. Hematoma was located on the left side in two cases. Average hematoma volume was 50.8 mL (38–63.7 mL), which was estimated by head CT scan. Hematoma distribution was lateral type with ventricular perforation in both cases. Both cases marked 3 points in ICH score. In each patient, almost all of the hematoma in the thalamus was removed. Intraventricular hematoma was also removed as much as possible under the endoscopic approach area. The average operation time was 76 minutes (ranging from 60 to 92 minutes).
The level of consciousness was improved in the early postoperative period. Mean level of consciousness after hematoma removal was 9.5 points with GCS. Mean clinical outcomes at the one month after surgery were 4.5 points for mRS and 2.5 points for GOS.
DISCUSSION
Improvement in consciousness was observed promptly after hematoma evacuation. Because of preserved general neurological dysfunction, improvement in mRS and GOS at one month was insufficient.
Endoscopic hematoma removal is less invasive to the brain, and its indications are expanding. However, because the thalamus is located deep in the brain, it requires a longer penetration distance through the normal brain to reach the hematoma. Therefore, various nerve fibers are easily damaged and neurological dysfunction is likely to remain severe.
In the early days of endoscopic surgery, Hsieh proposed an approach from the frontal lobe, similar to stereotactic hematoma removal or craniotomy [13]. However, the distance from the brain surface to the hematoma is longer, and the brain damage associated with the surgery is greater. In addition, the lateral part of the hematoma is difficult to remove. Various approaches have been reported to compensate for these disadvantages. Chen proposed an approach from the occipital lobe [14]. The posterior approach requires neck extension and rotation, which easily complicates the surgical position. In addition, if there is no expansion of the lateral ventricles, especially in the posterior horn, it requires a long tract in the occipital lobe to the ventricular peritoneal puncture. Miki also mentioned the availability of endoscopic hematoma removal from the thalamus through the posterior approach [24]. Xie also shows the superiority of the trans-pineal method [25]. The approach is from the wall of the third ventricle, and posterior and lateral hematomas are easily visible. However, careful orientation may be necessary because the approach passes close to the posterior perfusion vein. Related to this approach, Sun proposed the transcortical-transventricular method [26]. This procedure is useful for medial type hemorrhage with much hematoma in the third ventricle. However, lateral type hematomas distributed far lateral from the ventricle may be difficult to approach with endoscopic devices.
Based on the previous researches, the trans-temporal lobe approach was considered as the next candidate root toward the middle cerebral area. The approach from the middle temporal gyrus is a procedure that can be performed from the non-eloquent area. Yamazaki proposed this approach for capsular hematoma removal [27]. They presented desirable hematoma removal by trans-middle temporal gyrus method. Different from their approach tract, here presented approach takes the direction shifted to caudal than that to the capsular. This makes it easier to secure the visual axis and establish the surgical field.
Especially, thalamic hemorrhage with posterolateral type or global type in Chung’s classification is applicable for this approach. Because this procedure requires a surgical tract in the enlarged white matter from the middle temporal gyrus toward the thalamus, the upward displacement of the Sylvian fissure by the protruding hemorrhage facilitates access to the hematoma. In addition, both posterolateral and global hemorrhages are associated with high mortality and morbidity. Less invasive hematoma removal is urgently needed.
This procedure could be the next generation approach against severe thalamic hemorrhage.
In addition, this surgical approach has several advantages as a minimally invasive procedure. First, only a small skin incision is required for 1 burr hole perforation. Second, the distance from the brain surface to the hematoma is short, resulting in minimal brain damage. In addition, since it passes from the brain surface to the hematoma only through non-eloquent areas, there is less disruption associated with surgical invasion. Therefore, this procedure takes less time than surgical craniotomy.
In the future advanced, local anesthesia may be used to treat patients who are in poor general condition or have impaired circulatory or respiratory functions that make general anesthesia unsafe.
On the other hand, there are some disadvantages to this method of surgery.
First, it requires careful planning to determine the direction of the endoscope puncture. Therefore, the use of a navigation system is desirable, which requires more time to prepare for emergency surgery. Second, it is easy to become disoriented during the hematoma removal operation. Disorientation of approach direction and point is caused by oblique head position. Navigation system supports guidance to the hematoma and avoidance of surrounding eloquent area. At third, technical skill is required. In this procedure, the use of a thin rigid sheath is desirable, which makes it easier to perform the following operations including suction washing and removal of hematoma. Therefore, familiarity with endoscopic-assisted surgical techniques such as hematoma aspiration, washing, and hemostasis is required. Hematoma distribution in the medial region and intraventricular perforation are prone to inadequate hematoma removal, and require careful determination of the indication for treatment.
The transcortical tract from the middle temporal gyrus to the thalamic hematoma is surrounded by several functionally important nerve fibers. Therefore, it is very important and difficult to determine the puncture point and directional trajectory to the hematoma.
Complications of puncture site displacement include the following. If the shift is cranial, the superior temporal gyrus is easily injured, resulting in aphasia and cognitive dysfunction. In contrast, caudal or dorsal displacement tends to damage the Labbe’s vein on the surface of the brain. If ventral shift to the tip of the temporal lobe is more likely to cause cognitive dysfunction. In contrast, dorsal shift is more likely to cause aphasia, especially on the left side, due to its proximity to the Wernicke’s area. Other complications of directional shift in the direction of the hematoma include the following. With cranial or ventral shift, the pyramidal tracts including the internal capsule are damaged, and motor paralysis is likely to occur laterally. In contrast, caudal shift damages the optic radiation and causes contralateral visual field defects. Excessive caudal shift damages the hippocampal amygdala, causing memory impairment and emotional dysphoria. If the shift is from caudal to ventral, it is more likely to cause damage to the hypothalamus, midbrain, and prolonged loss of consciousness due to reticular damage.
Cerebral structural factors also adversely affect this approach. Because of elderly patients usually have cerebral atrophy, the approach tract of the endoscope may pass through the wide Sylvian fissure. In such cases, it is necessary to advance the sheath into the hematoma cavity under careful endoscopic observation without damaging the middle cerebral artery (MCA). In these patients, additional care must be taken to avoid moving the sheath as much as possible during removal of the hematoma. Otherwise, the sheath may injure the MCA or its perforator artery with subarachnoid hemorrhage.
Reservation of neural function is urgently required. However, it is difficult to confirm the tractography before surgical treatment because the operation is often urgent and accompanied by nerve palsy due to hematoma or surrounding edema. Therefore, it is difficult to accurately estimate the nerve damage caused by the surgical procedure. As a result, the degree of improvement of neurological symptoms and the degree of residual disability cannot be adequately assessed.
Improving the prognosis for activities of daily living is also important. Long-term disability of impaired consciousness and functional impairment due to thalamic hemorrhage lead to progressive disuse syndrome, long-term bedriddenness, and increased medical care costs, including intubated feeding management. Therefore, early removal of the hematoma will relieve pressure on the reticular formation and edematous changes, and promote rehabilitation by facilitating improvement. Hematoma removal may promote recovery of motor function by relieving pressure on the pyramidal tract. In addition, it may leave space to promote improvement in aphasia, visual field abnormalities, and cognitive dysfunction. It seems important to reduce the waste of social and medical resources and to improve not only mortality but also morbidity.
The number of cases in this study was small, and one patient did not achieve adequate recovery of functional prognosis. However, with further accumulation of clinical cases and improvement of surgical techniques, this study may contribute to the good prognosis of severe thalamic hemorrhage.
CONCLUSION
The surgical procedure described in this report represents a minimally invasive and useful method for evacuating a thalamic hematoma by endoscopic intervention.
REFERENCES
1.
Kumral E, Kocaer T, Ertübey NO, Kumral K. Thalamic hemorrhage. A prospective study of 100 patients. Stroke 1995;26(6):964–70. [CrossRef]
[Pubmed]

2.
Schmahmann JD. Vascular syndromes of the thalamus. Stroke 2003;34(9):2264–78. [CrossRef]
[Pubmed]

3.
Schlunk F, Greenberg SM. The pathophysiology of intracerebral hemorrhage formation and expansion. Transl Stroke Res 2015;6(4):257–63. [CrossRef]
[Pubmed]

4.
Lee TH. Intracerebral hemorrhage. Cerebrovasc Dis Extra 2025;15(1):1–8. [CrossRef]
[Pubmed]

5.
Lee SH, Park KJ, Kang SH, Jung YG, Park JY, Park DH. Prognostic factors of clinical outocomes in patients with spontaneous thalamic hemorrhage. Med Sci Monit 2015;21:2638–46. [CrossRef]
[Pubmed]

6.
Teo KC, Fong SM, Leung WCY, et al. Location-specific hematoma volume cutoff and clinical outcomes in intracerebral hemorrhage. Stroke 2023;54(6):1548– 57. [CrossRef]
[Pubmed]

7.
Kwak R, Kadoya S, Suzuki T. Factors affecting the prognosis in thalamic hemorrhage. Stroke 1983;14(4):493–500. [CrossRef]
[Pubmed]

8.
Ali M, Zhang X, Ascanio LC, et al. Long-term functional independence after minimally invasive endoscopic intracerebral hemorrhage evacuation. J Neurosurg 2022;138(1):154–64. [CrossRef]
[Pubmed]

9.
Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): A randomised trial. Lancet 2005;365(9457):387–97. [CrossRef]
[Pubmed]

10.
Mendelow AD, Gregson BA, Rowan EN, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): A randomised trial. Lancet 2013;382(9890):397–408. [CrossRef]
[Pubmed]

11.
Scaggiante J, Zhang X, Mocco J, Kellner CP. Minimally invasive surgery for intracerebral hemorrhage. Stroke 2018;49(11):2612–20. [CrossRef]
[Pubmed]

12.
13.
Hsieh PC. Endoscopic removal of thalamic hematoma: A technical note. Minim Invasive Neurosurg 2003;46(6):369–71. [CrossRef]
[Pubmed]

14.
Chen CC, Lin HL, Cho DY. Endoscopic surgery for thalamic hemorrhage: A technical note. Surg Neurol 2007;68(4):438–42. [CrossRef]
[Pubmed]

15.
Suyama D, Kumar B, Watanabe S, et al. Endoscopic approach to thalamic and interventricular hemorrhage. Asian J Neurosurg 2019;14(1):77–81. [CrossRef]
[Pubmed]

16.
Shimizu Y, Tsuchiya K, Fujisawa H. Endoscopic surgery for thalamic hemorrhage with intraventricular hemorrhage: Effects of combining evacuation of a thalamic hematoma to external ventricular drainage. Asian J Neurosurg 2019;14(4):1112–5. [CrossRef]
[Pubmed]

17.
Fu CH, Wang N, Chen HY, Chen QX. Endoscopic surgery for thalamic hemorrhage breaking into ventricles: Comparison of endoscopic surgery, minimally invasive hematoma puncture, and external ventricular drainage. Chin J Traumatol 2019;22(6):333–9. [CrossRef]
[Pubmed]

18.
Chen KY, Kung WM, Kuo LT, Huang AP. Ultrarapid endoscopic-aided hematoma evacuation in patients with thalamic hemorrhage. Behav Neurol 2021;2021:8886004. [CrossRef]
[Pubmed]

19.
Song R, Ali M, Pan J, et al. Functional outcome after minimally invasive endoscopic evacuation of thalamic intracerebral hemorrhage. World Neurosurg 2021;149:e592–9. [CrossRef]
[Pubmed]

20.
Sun E, Lu S, Chen B, Wu Q. An endoscopic-assisted contralateral paramedian supracerebellar infratentorial approach in the treatment of thalamic hemorrhage with hematoma extension into the brainstem: A case report. Front Surg 2023;10:1277990. [CrossRef]
[Pubmed]

21.
Chung CS, Caplan LR, Han W, Pessin MS, Lee KH, Kim JM. Thalamic haemorrhage. Brain 1996;119 (Pt 6):1873–86. [CrossRef]
[Pubmed]

22.
23.
Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32(4):891–7. [CrossRef]
[Pubmed]

24.
25.
Xie T, Liu S, Zhang X, Yang L, Liu T, Chen P, Li Z. Endoscopic supracerebellar infratentorial transpineal approach for posterior-medial thalamic lesions: Surgical technique and clinical experience. Oper Neurosurg (Hagerstown) 2024;27(2):187–93. [CrossRef]
[Pubmed]

26.
Sun H, Wang Y, Yu S, Li Z, Wang T. Endoscopic-assisted translateral ventricular transchoroidal fissure approach for evacuation of medial-type thalamic hemorrhage: Case series. World Neurosurg 2020;143:183–9. [CrossRef]
[Pubmed]

27.
Yamazaki K, Ogiwara T, Kitamura S, et al. Endoscopic evacuation of putaminal hemorrhage using the transmiddle temporal gyrus approach: Technical notes and case presentation. J Neurol Surg A Cent Eur Neurosurg 2024;85(5):520–5. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Acknowledgments
The author thanks Dr. Ken Kazumata for his useful suggestion and technical advises for this research. Much informative discussion with him contributed to establish this technical protocol.
Author ContributionsAkira Tempaku - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthor declares no conflict of interest.
Copyright© 2025 Akira Tempaku. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.